Revolutionizing Diagnostics: The Power of CLIA Diagnostic Kits
Introduction: In the ever-evolving field of healthcare, accurate and rapid diagnosis is key to effective treatment. Among the various diagnostic technologies, CLIA (Chemiluminescence Immunoassay) diagnostic kits have emerged as a game-changer, offering unmatched precision and speed in detecting a wide range of diseases. From infectious diseases to hormonal disorders, CLIA diagnostic kits are transforming the way healthcare professionals approach diagnostics.

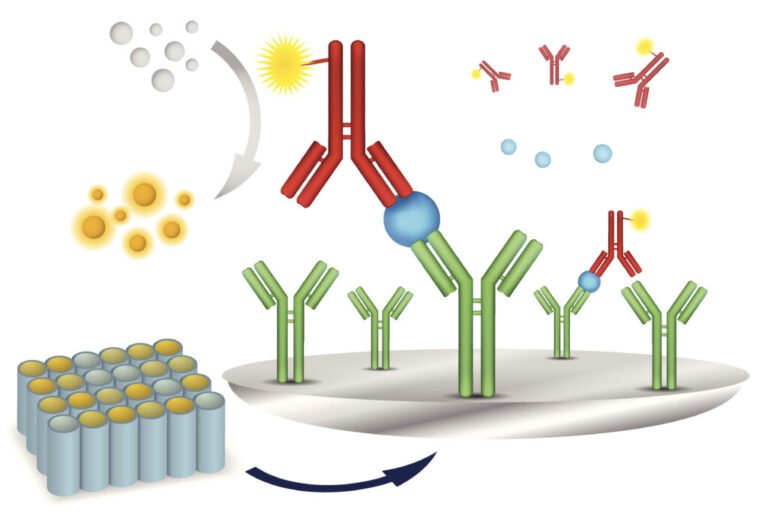
What is CLIA? Chemiluminescence Immunoassay (CLIA) is a sophisticated diagnostic technique that combines immunology and chemiluminescence. It involves using antibodies to detect specific antigens or proteins in a patient’s sample, such as blood or urine. The detection is made possible through a chemiluminescent reaction, where light emission is measured to determine the presence and concentration of the target analyte. This method is highly sensitive, making it ideal for detecting even minute amounts of biomarkers.
Advantages of CLIA Diagnostic Kits:
- High Sensitivity and Specificity: CLIA diagnostic kits are renowned for their ability to detect low concentrations of biomarkers with exceptional accuracy. This high sensitivity and specificity reduce the chances of false positives and false negatives, ensuring reliable results for both patients and healthcare providers.
- Rapid Results: Time is often critical in medical diagnosis, and CLIA kits deliver results quickly. This allows for faster decision-making, which is crucial in emergencies and for early-stage disease detection. The rapid turnaround time also enhances patient satisfaction and improves the overall efficiency of healthcare services.
- Wide Range of Applications: CLIA diagnostic kits are versatile and can be used to diagnose various conditions, including infectious diseases (e.g., COVID-19, HIV), autoimmune disorders, cardiovascular diseases, cancer markers, and hormone imbalances. This broad applicability makes them an indispensable tool in clinical laboratories.
- Automation and Scalability: Many CLIA systems are automated, allowing for high-throughput testing. This automation reduces human error, streamlines workflow, and makes it possible to process a large number of samples simultaneously. The scalability of CLIA systems also makes them suitable for both small clinics and large reference laboratories.
- Cost-Effective: Despite their advanced technology, CLIA diagnostic kits offer a cost-effective solution for healthcare providers. Their accuracy reduces the need for repeat tests, and their efficiency lowers labor and operational costs. This makes CLIA a cost-efficient choice for both patients and healthcare facilities.
Applications in Disease Detection:
- Infectious Diseases: CLIA kits have played a pivotal role in the fight against infectious diseases. For example, during the COVID-19 pandemic, CLIA diagnostic kits were crucial in rapidly detecting the virus, enabling timely intervention and controlling the spread of the disease.
- Cancer Diagnostics: Early detection of cancer can significantly improve treatment outcomes. CLIA diagnostic kits can detect specific cancer markers, allowing for early diagnosis and monitoring of treatment efficacy.
- Hormonal and Metabolic Disorders: Hormonal imbalances can lead to various health issues. CLIA kits are used to measure hormone levels accurately, aiding in the diagnosis and management of conditions like thyroid disorders, diabetes, and reproductive health issues.
Conclusion:
CLIA diagnostic kits are at the forefront of modern diagnostics, offering unparalleled accuracy, speed, and versatility. As technology continues to advance, these kits will play an increasingly vital role in healthcare, improving patient outcomes and making diagnostics more accessible. Whether in large hospitals or small clinics, CLIA diagnostic kits are set to revolutionize the way we approach disease detection and management.