Why We Recommend Line Immunoassay (LIA) Over Skin Prick Testing for Allergy Diagnosis
Powered by Amindo Biologics’ laboratory-grade solutions
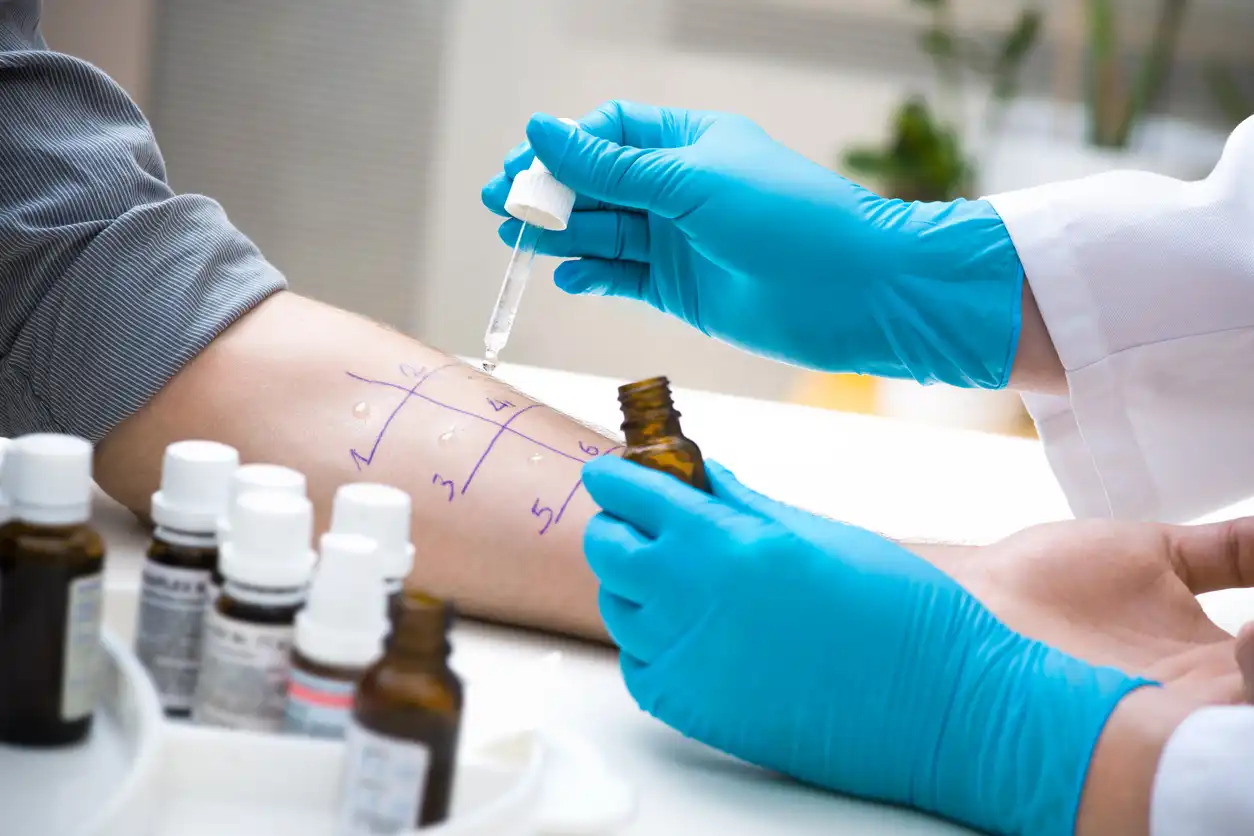
Allergy diagnosis has two common paths: traditional skin prick testing (SPT) performed on the forearm/back, and in-vitro blood testing, such as Line Immunoassay (LIA) that detects allergen-specific IgE in the lab. While SPT has been used for decades, many clinics and patients today prefer LIA because it’s cleaner, safer for at-risk patients, and delivers standardized, reproducible results—without interrupting medications.
Below, we explain the key differences and why our recommendation is to use LIA panels supplied by Amindo Biologics for most routine and advanced allergy workups.
The Limitations and Risks of Skin Prick Testing (SPT)
SPT can be useful in select settings, but it has several practical and clinical drawbacks you should consider:
- Risk of systemic reactions (rare but serious): Although uncommon, SPT can trigger generalized reactions in highly sensitized individuals. Clinics must keep emergency anaphylaxis medication and observation facilities ready.
- Not ideal for many patients: SPT is often unsuitable when the patient has dermatographism, active eczema/psoriasis on test sites, extensive skin disease, or poorly controlled asthma. It’s also typically avoided during pregnancy and used cautiously in those with a history of severe anaphylaxis.
- Requires antihistamine washout: Patients usually need to stop antihistamines and certain psychotropics for days in advance. This is inconvenient and may worsen symptoms.
- Operator and extract variability: Results depend on technique (depth, spacing, timing of reading) and the quality/freshness of allergen extracts, which can vary across clinics and over time.
- On-site time and discomfort: Multiple pricks (often 20–60) can be uncomfortable, especially for children; results must be read within a fixed time window in the clinic.
Why Line Immunoassay (LIA) is the Better Everyday Choice
Line Immunoassay is an in vitro method that measures allergen-specific IgE from a small blood sample. It brings clear advantages for patients, clinicians, and laboratories:
- Safer and medication-friendly
No direct allergen exposure on the skin and no antihistamine washout required. Suitable for patients with skin disease, children, the elderly, and those at higher risk for systemic reactions. - Standardized, objective results
Laboratory calibration minimizes operator bias. Results are quantitative/semiquantitative (class levels), easy to track over time, and compatible with quality systems (e.g., IQC/EQAS). - Broad, multiplex coverage
Test dozens of inhalant, food, and occupational allergens on a single strip—ideal for first-line screening, complex polysensitization, and longitudinal monitoring. - Efficient workflow
Batch processing reduces cost per result in active labs, with clean documentation for EMR/LIS integration and clear reports for patient counseling. - Better for follow-up and therapy planning
Comparable baseline units enable trend analysis (e.g., before and after avoidance measures, immunotherapy, or season changes).
Bottom line: LIA delivers safer, standardized, and scalable allergy diagnostics—without the practical hurdles of skin testing.
Where LIA Excels (Use Cases)
- Patients who cannot stop antihistamines or other interfering drugs
- Dermatological conditions or widespread rashes where SPT sites aren’t viable
- Pediatrics and needle-phobic patients who benefit from a single, brief blood draw
- Polysensitized patients needing a broad allergen overview in one run
- Clinics and labs requiring traceable, auditable results for quality programs
About Amindo Biologics’ Line Immunoassay Panels
Amindo Biologics provides a comprehensive LIA portfolio designed for Indian clinical practice and environmental exposures:
- Panels available: Inhalants (pollens, mites, molds, animal danders), Foods (milk/egg, nuts, seafood, cereals, fruits/vegetables), Mixed screening strips, and customizable add-ons.
- Reporting: Clear class levels with cutoff guidance and interpretive comments that support clinical decisions.
- Quality & workflow: Built for routine labs and reference centers, with robust controls, streamlined incubation/wash steps, and LIS-friendly output.
- Clinical support: Training for technicians, result interpretation aids, and after-sales service to keep throughput and consistency high.
Disclaimer
This article is intended for healthcare professionals and laboratory managers. Clinical decisions should consider patient history, exam findings, and local guidelines.