Why Congenital Adrenal Hyperplasia (CAH) Remains a Silent Neonatal Emergency?
17-OHP ELISA is the mandatory first-tier newborn screen for Congenital Adrenal Hyperplasia(CAH). Learn diagnostic workflows, cutoffs, false positives & how Amindo enables life-saving NICU screening.
The Neonatal Crisis That Rarely Announces Itself
Neonatal medicine is uniquely unforgiving. Physiology is fragile, compensatory reserves are limited, and the margin between clinical stability and collapse is narrow. Among disorders that exploit this narrow margin, Congenital Adrenal Hyperplasia (CAH) is one of the most clinically dangerous — not because it is rare, but because it is biochemically silent until catastrophic. CAH due to 21-hydroxylase deficiency is the most common classic form and underlies the vast majority of salt-wasting presentations. NCBI
In many NICUs, CAH is not “missed” — it is misclassified as sepsis, dehydration, feeding intolerance, or electrolyte imbalance until salt-wasting adrenal collapse is already underway. The difference between an uneventful neonatal course and sudden infant death is often determined by a single biochemical gate: 17-hydroxyprogesterone (17-OHP).
A Clinical Pattern Every Neonatologist Recognizes
Image source: https://diag.vn/en/thongtinyte/the_17_oh_progesterone/
A 9-day-old infant presents with vomiting and lethargy.
Serum sodium is low. Potassium is high.
He appears septic.
Broad-spectrum antibiotics are initiated.
Intravenous fluids are started.
Cultures are sent.
Nothing grows. Yet the infant worsens.
A 17-hydroxyprogesterone level is finally requested.
The result is markedly elevated.
Hydrocortisone and fludrocortisone are started.
Within hours:
• Vomiting resolves
• Electrolytes normalize
• Hemodynamic stability returns
It was not sepsis.
It was salt-wasting CAH.
This sequence is not rare. It is simply under-documented. Early biochemical screening would have prevented crisis and hospital readmissions.
What 17-OHP Actually Represents
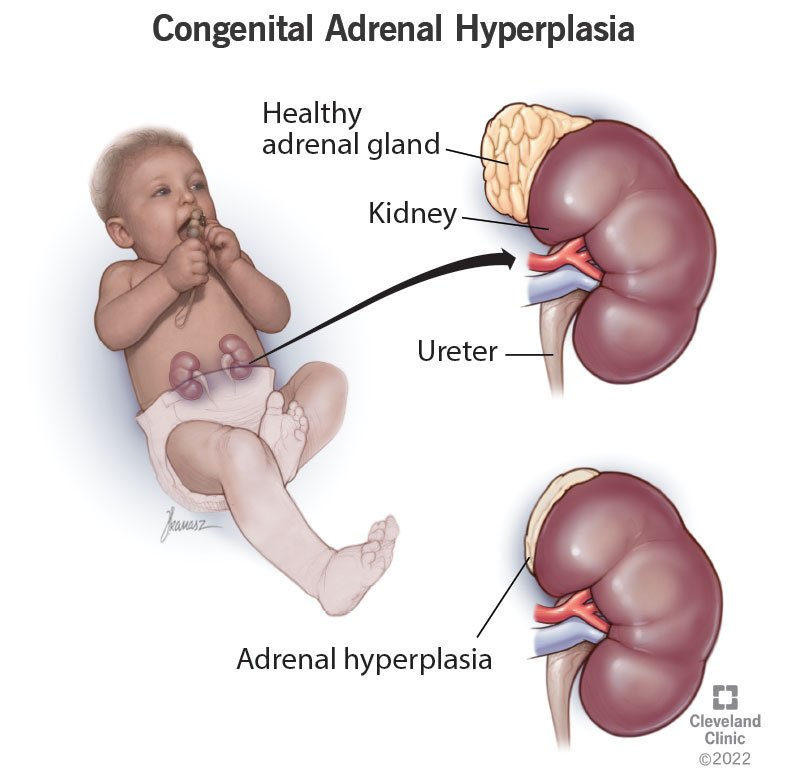
17-hydroxyprogesterone is a steroid precursor in the adrenal cortisol biosynthesis pathway.
In healthy neonates it is transiently elevated at birth and rapidly normalizes. In 21-hydroxylase deficiency (≈95% of classic CAH cases), cortisol and aldosterone synthesis are impaired, adrenocorticotropic hormone (ACTH) rises, and 17-OHP accumulates. This accumulation is not a secondary marker — it is the earliest detectable biochemical footprint of life-threatening adrenal failure. PMC+1
That is why 17-OHP is not a routine endocrine panel test.
It is a screening gate.
Congenital Adrenal Hyperplasia: Why It Is Lethal in Newborns

Classic CAH is characterized by:
| Defect | Physiological Result | Clinical Consequence |
| ↓ Cortisol | Impaired stress response | Sepsis-like collapse |
| ↓ Aldosterone | Renal salt loss | Dehydration & shock |
| ↑ ACTH | Adrenal hyperplasia | Hormonal imbalance |
| ↑ Androgens | Virilization | Ambiguous genitalia |
Salt-wasting CAH, constituting approximately 70–75% of classic cases, typically declares itself between days 5–14 of life — precisely when many neonates have already been discharged. Indian Pediatrics+1
By the time electrolyte abnormalities are recognized, adrenal crisis is often already in progress.
Epidemiology: CAH Is More Common Than Clinicians Assume
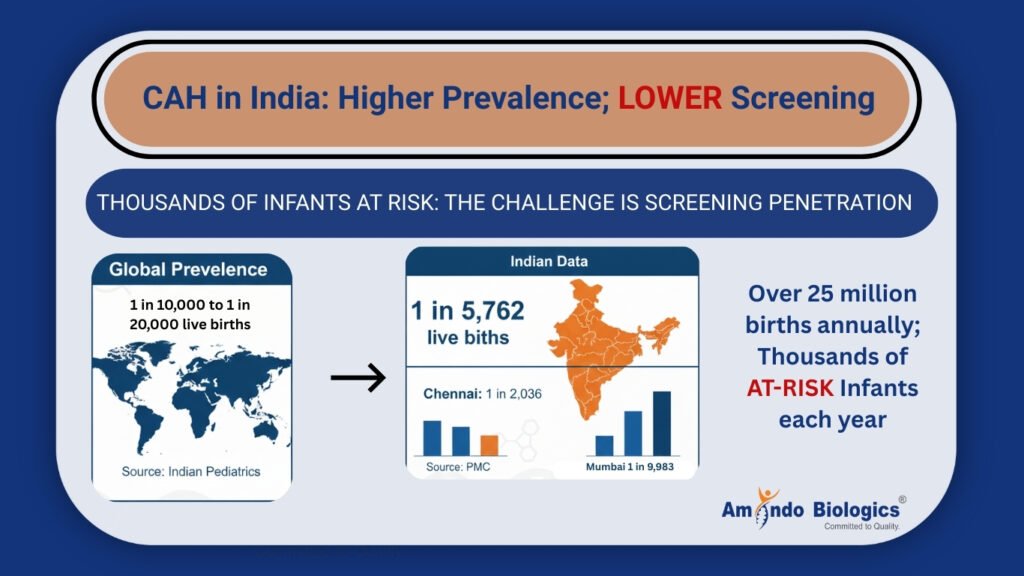
Traditional global prevalence estimates range from approximately 1 in 10,000 to 1 in 20,000 live births. Indian Pediatrics
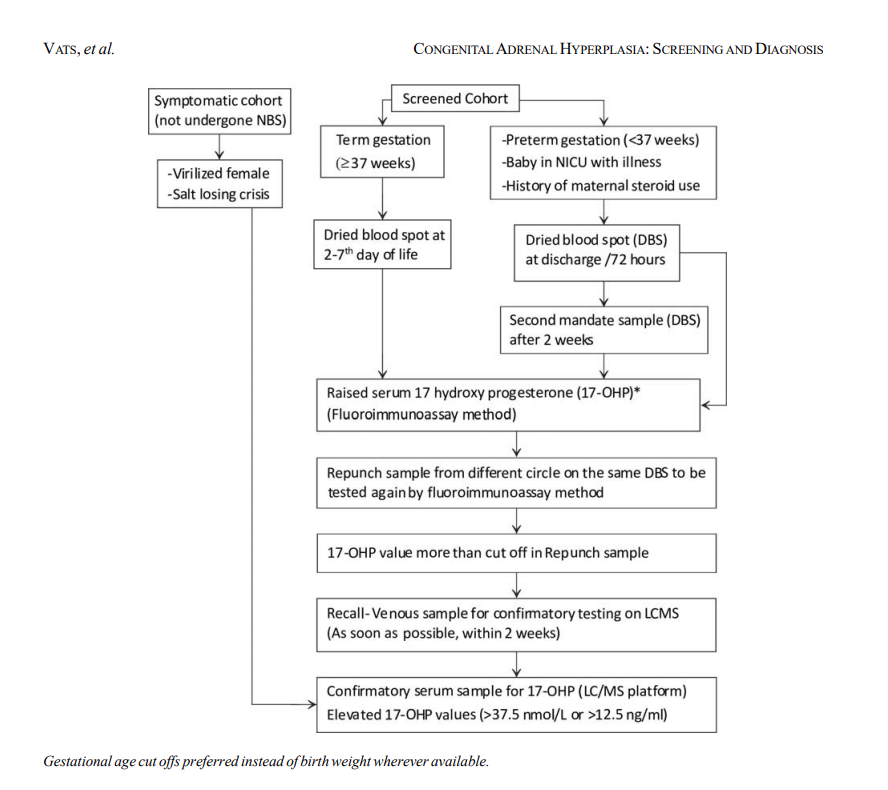
Fig: Suggested algorithm for screening of infants for congenital adrenal hyperplasia.(Source:https://www.indianpediatrics.net/jan2020/49.pdf?utm_source=chatgpt.com)
However, Indian newborn screening cohorts show a screen-positive rate of about 1 in 5,762 births, with considerable regional differences (e.g., 1:2,036 in Chennai vs 1:9,983 in Mumbai). PMC
Given India’s high birth volume — over 25 million annually — this translates into thousands of at-risk infants each year. The problem is not rarity — it is screening penetration. Indian Pediatrics
Why Is 17-OHP Screening Mandatory, Not Optional?
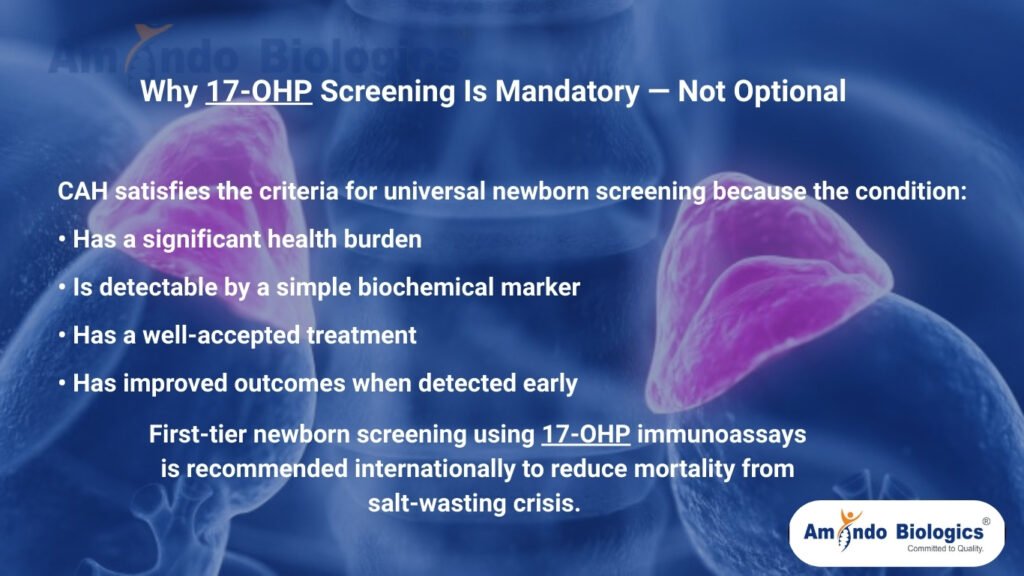
CAH satisfies the criteria for universal newborn screening because the condition:
• Has a significant health burden
• Is detectable by a simple biochemical marker
• Has a well-accepted treatment
• Has improved outcomes when detected early
First-tier newborn screening using 17-OHP immunoassays is recommended internationally to reduce mortality from salt-wasting crisis. Lippincott Journals
Without screening, clinical detection often comes too late.
The Preterm False-Positive Problem and Why It Is Not a Reason to Avoid Screening
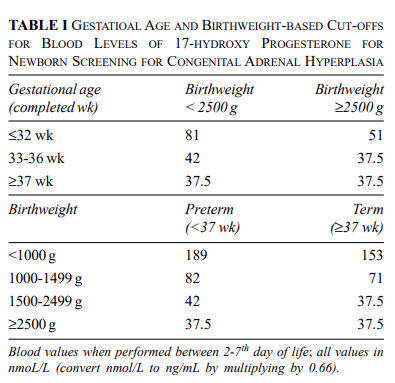
Fig: GESTATIONAL AGE AND BIRTHWEIGHT-BASED CUT-OFFS FOR BLOOD LEVELS OF 17-HYDROXY PROGESTERONE FOR NEWBORN SCREENING FOR CONGENITAL ADRENAL HYPERPLASIA ( Source: https://www.indianpediatrics.net/jan2020/49.pdf?utm_source=chatgpt.com)
Preterm and low-birth-weight infants have physiologically higher 17-OHP concentrations due to adrenal immaturity, leading to elevated false-positive rates in standard immunoassays. PMC+1
However, this is not a flaw in 17-OHP screening — it is a limitation of unstructured workflows. Modern programs reduce false positives by:
• Gestational age-adjusted cutoffs
• Birthweight-stratified reference intervals
• Reflex confirmatory testing
Such structured strategies maintain high sensitivity while improving specificity.
What Is The Correct Diagnostic Architecture?
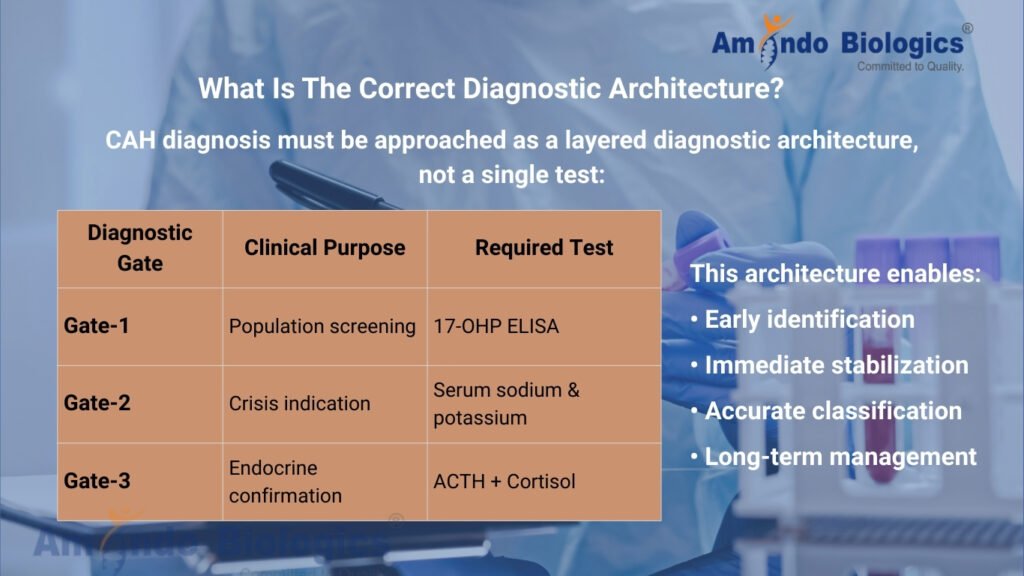
CAH diagnosis must be approached as a layered diagnostic architecture, not a single test:
| Diagnostic Gate | Clinical Purpose | Required Test |
| Gate-1 | Population screening | 17-OHP ELISA |
| Gate-2 | Crisis indication | Serum sodium & potassium |
| Gate-3 | Endocrine confirmation | ACTH + Cortisol |
This architecture enables:
• Early identification
• Immediate stabilization
• Accurate classification
• Long-term management
Why ELISA Is the Appropriate Screening Platform?
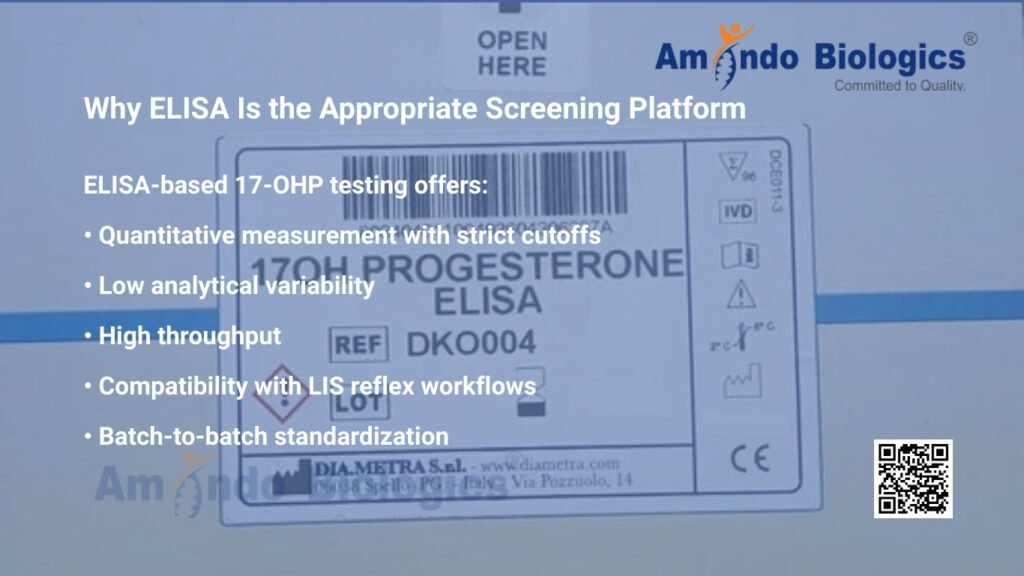
ELISA-based 17-OHP testing offers:
• Quantitative measurement with strict cutoffs
• Low analytical variability
• High throughput
• Compatibility with LIS reflex workflows
• Batch-to-batch standardization
These attributes are essential for systematic newborn screening programs that demand precision and reproducibility.
What Are The Clinical and Health-System Impact?

For the Infant
• Prevents adrenal crisis
• Prevents sudden infant death
• Preserves growth and development
• Avoids unnecessary ICU admissions
For the Hospital
• Reduces unexplained NICU emergencies
• Shortens NICU stay duration
• Reduces unnecessary sepsis protocols
• Improves medico-legal defensibility
Structured CAH screening reduces morbidity, cost, and clinical burden.
Amindo Endocrine Diagnostic Infrastructure

Amindo Biologics supports structured newborn endocrine diagnostics through:
• 17-OHP ELISA – First-tier CAH screening
• ACTH – Confirmation and staging
• Cortisol – Functional adrenal evaluation
We do not supply isolated hormone kits.
We architect neonatal diagnostic safety nets.
CAH does not kill because it is aggressive —
It kills because it is not screened.
17-OHP is the biochemical gate that converts CAH from a hidden neonatal emergency into a preventable diagnosis.
Every NICU without structured 17-OHP screening is operating without a neonatal safety net.
Clinical Ethics Disclaimer
This content is intended for professional education and does not replace institutional newborn screening protocols or clinical judgment.
FAQs:
FAQ 1
Why should 17-OHP be included as a routine NICU screening test?
Because salt-wasting CAH clinically mimics sepsis and dehydration and often presents after discharge.
17-OHP is the only validated biochemical marker capable of detecting this life-threatening condition before electrolyte collapse and shock occur.
FAQ 2
Why is ELISA the preferred platform for 17-OHP screening in routine laboratories?
ELISA provides quantitative cut-off based reporting, batch consistency, automation compatibility, and LIS reflex workflow support — making it ideal for population-level newborn screening programs where analytical reproducibility and interpretive stability are mandatory.
FAQ 3
What Amindo products support complete CAH diagnostic workflows?
| Diagnostic Gate | Amindo Product |
| Screening | 17-OHP ELISA |
| Confirmation | ACTH |
| Confirmation | Cortisol |
Together, these form a complete endocrine reflex architecture.
FAQ 4
Why are electrolytes alone insufficient to rule out CAH?
Electrolyte derangement appears after adrenal collapse has already begun.
17-OHP rises before clinical deterioration, making it a preventive screening gate rather than a late-stage crisis indicator.
FAQ 5
How does structured 17-OHP screening improve hospital outcomes and margins?
• Prevents recurrent NICU admissions
• Reduces prolonged sepsis workups
• Lowers antibiotic misuse
• Improves medico-legal defensibility
• Increases diagnostic yield per newborn sample
FAQ 6
Can 17-OHP screening be applied to preterm infants?
Yes — when gestational age or birth-weight stratified cut-offs are applied.
Amindo ELISA workflows support such stratified interpretation.
FAQ 7
Who should routinely order 17-OHP screening?
• NICUs
• Neonatologists
• Pediatricians
• Newborn screening laboratories
• Government screening programs
• Corporate hospital chains
FAQ 8
How does Amindo differentiate itself in endocrine diagnostics?
Amindo does not supply isolated hormone kits.
Amindo delivers complete newborn endocrine diagnostic architecture — screening, reflex, and confirmation — in a structured, standardized laboratory framework.
FAQ 9
Is CAH screening cost-effective?
Yes. The cost of one 17-OHP screen is negligible compared to the cost of NICU admissions, adrenal crisis management, and long-term morbidity due to delayed diagnosis.
FAQ 10
What is the single most important clinical takeaway?
17-OHP is not optional. It is the neonatal safety gate.
At Amindo Biologics, we enable structured, life-saving neonatal endocrine diagnostics.
Our 17-OHP ELISA, ACTH, and Cortisol assays provide standardized, high-performance newborn screening and reflex confirmation — supporting early detection of congenital adrenal hyperplasia, reduced diagnostic ambiguity, medico-legal defensibility, and confident NICU decision-making.
We do not supply kits.
We architect neonatal diagnostic safety nets. We protect newborn lives.